1. Laubscher M, Mitchell C, Timms A, et al. Outcomes following femoral lengthening. An initial comparison of the Precice intramedullary lengthening nail and the LRS external fixator monorail system. Bone Joint J 2016;98-B:1382-8.
2. Landge V, Shabtai L, Gesheff M, et al. Patient satisfaction after limb lengthening with internal and external devices. J Surg Orthop Adv 2015;24(3):174-9.
3. Richardson, S, Schairer W, Fragomen A, et al. Cost comparison of femoral distraction osteogenesis with external lengthening over a nail versus internal magnetic lengthening nail. J Am Acad Orthop Surg 2019;27(9):e430-e436.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.Proper surgical procedure is the responsibility of the medical professional. Operative techniques are furnished as an informative guideline. Each surgeon must evaluate the appropriateness of a technique based on his or her personal medical credentials and experience.
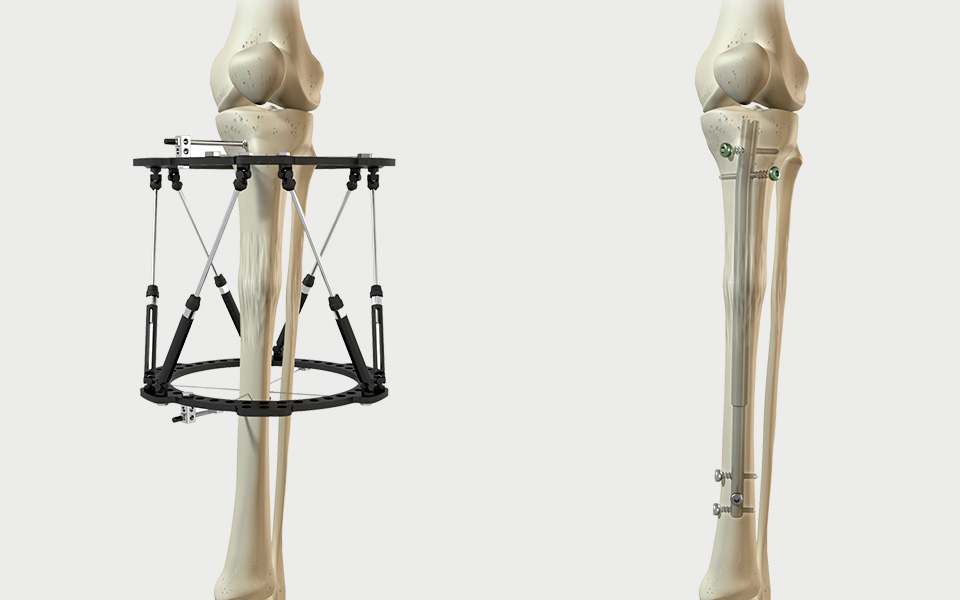
The PRECICE Intramedullary Limb Lengthening System is intended for limb lengthening, open and closed fracture fixation, pseudoarthrosis, mal-unions, non-unions, or bone transport of long bones in patients age 18 years and older.
Please refer to the Instructions For Use supplied with the product for specific information on the indications for use, contraindications, warnings, precautions, cautions and sterilization. Instructions For Use are also available by contacting us.