P360
P360 provides comprehensive pathology-driven solutions to help surgeons deliver patient-specific surgical plans while enhancing OR workflow and efficiency from the prone position.
P360 provides comprehensive pathology-driven solutions to help surgeons deliver patient-specific surgical plans while enhancing OR workflow and efficiency from the prone position.
The NuVasive Tube System (NTS) is engineered to elevate surgical workflow through efficient, controlled positioning and optimized access. As the MIS access component of our P360 portfolio, NTS provides comprehensive solutions for both TLIF and decompression, furthering our goal to help surgeons deliver patient-specific surgical plans with our pathology-driven solutions, all from the prone position.
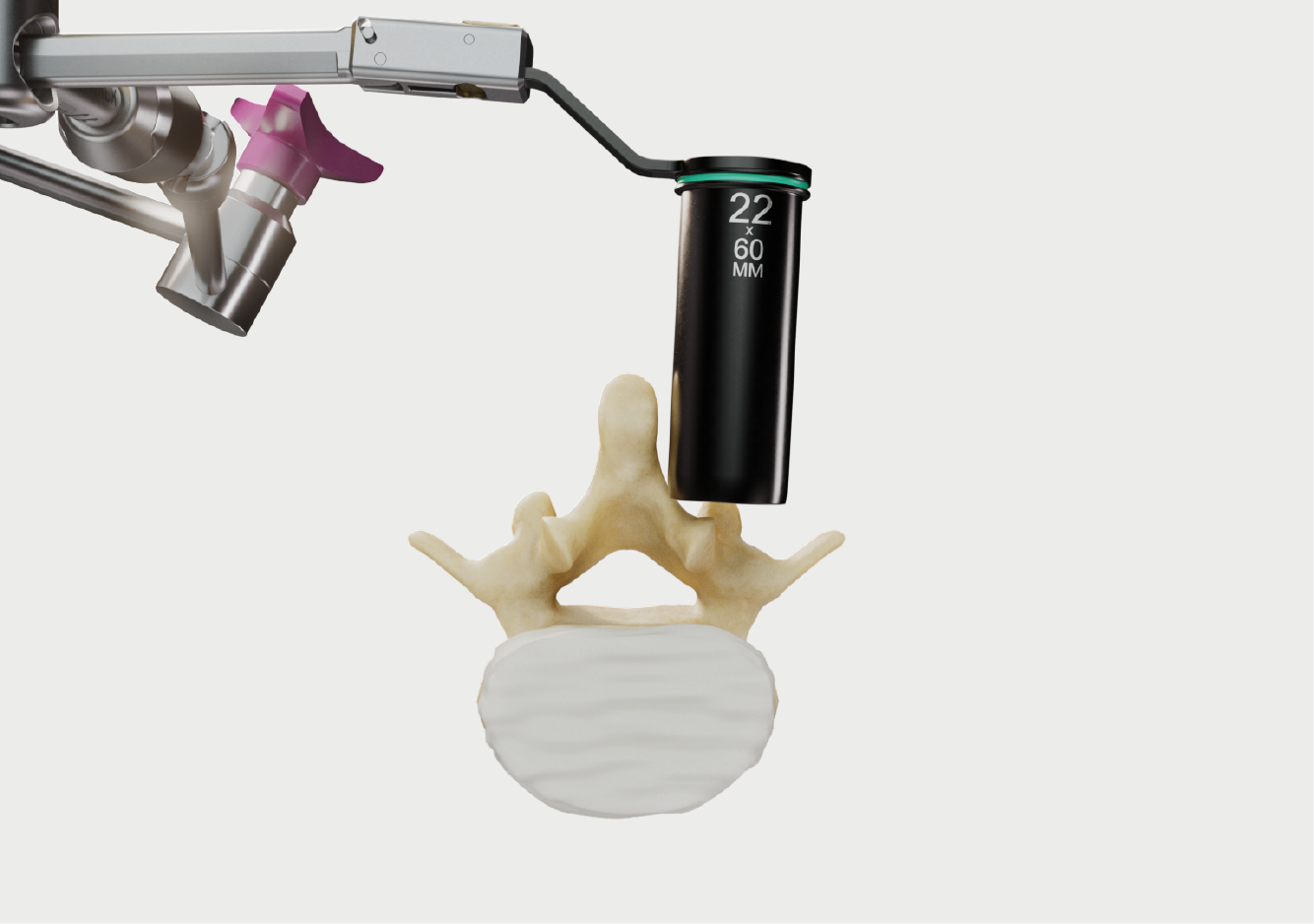
Seamless adjustment.
Increased cranial-caudal angulation and medial-lateral rotation.
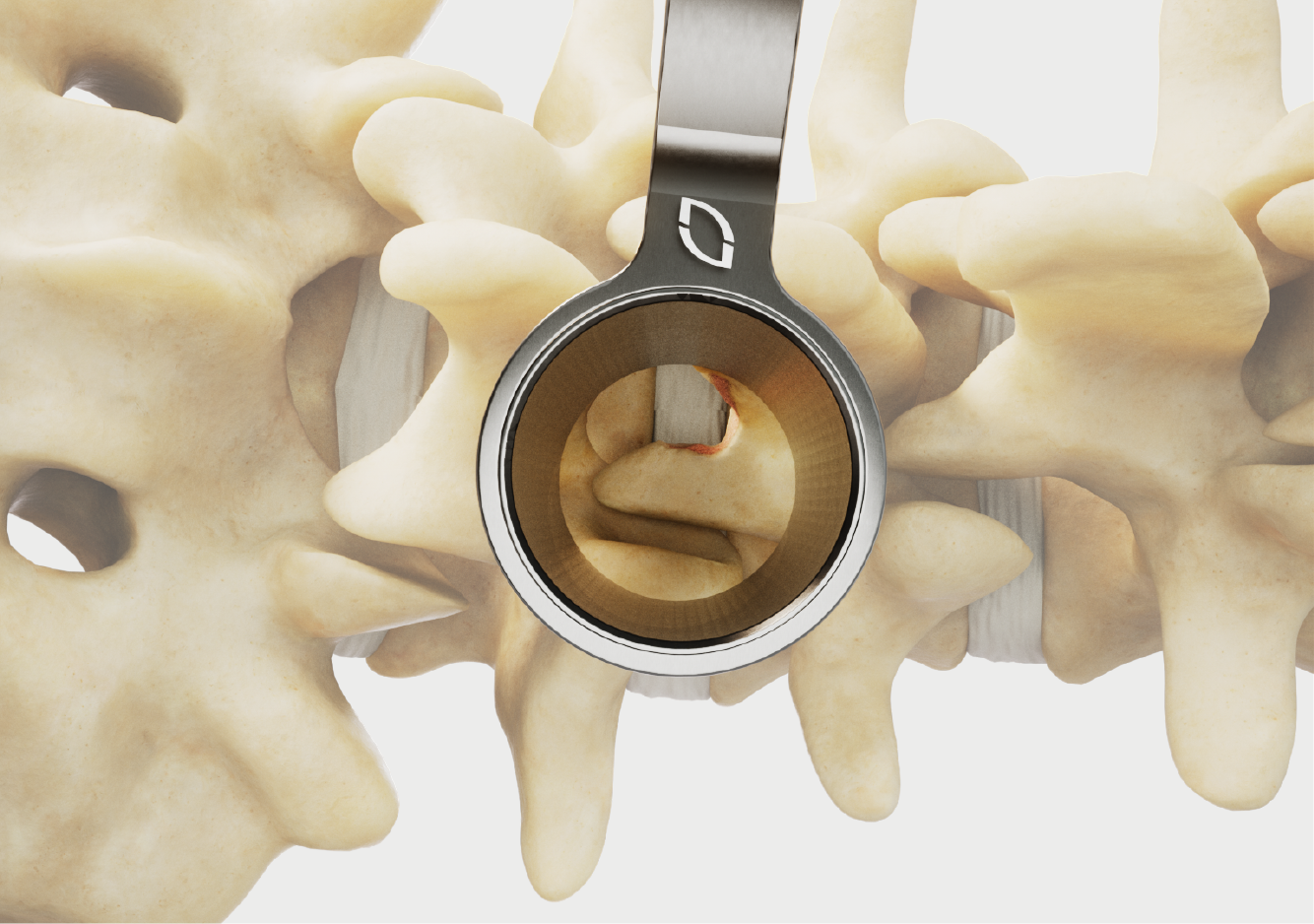
Standard and slotted tube options.
Diameters and lengths to address cervical to lumbar.
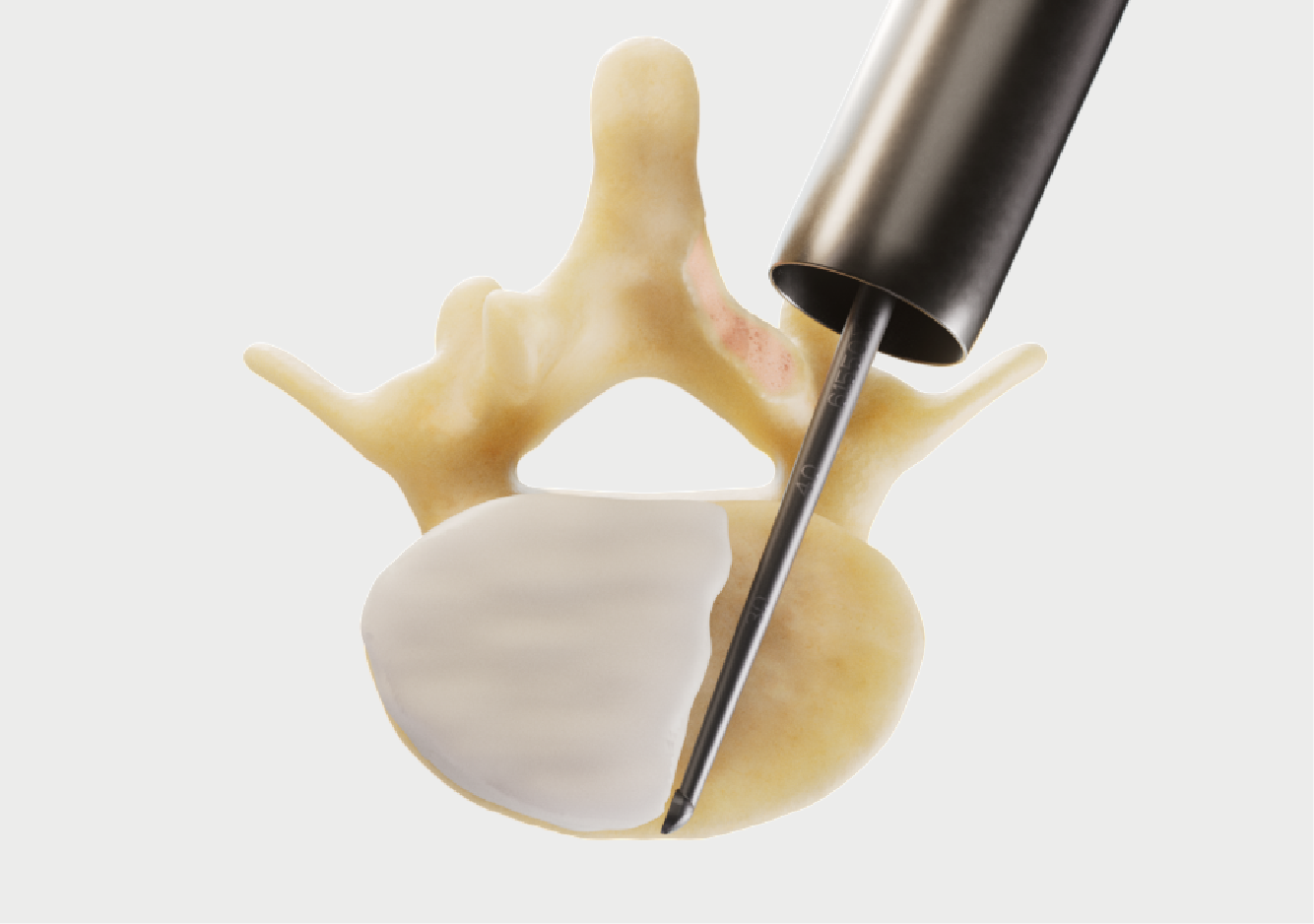
Complementary Micro Decompression instruments.
Thoughtfully designed for superior ergonomics.
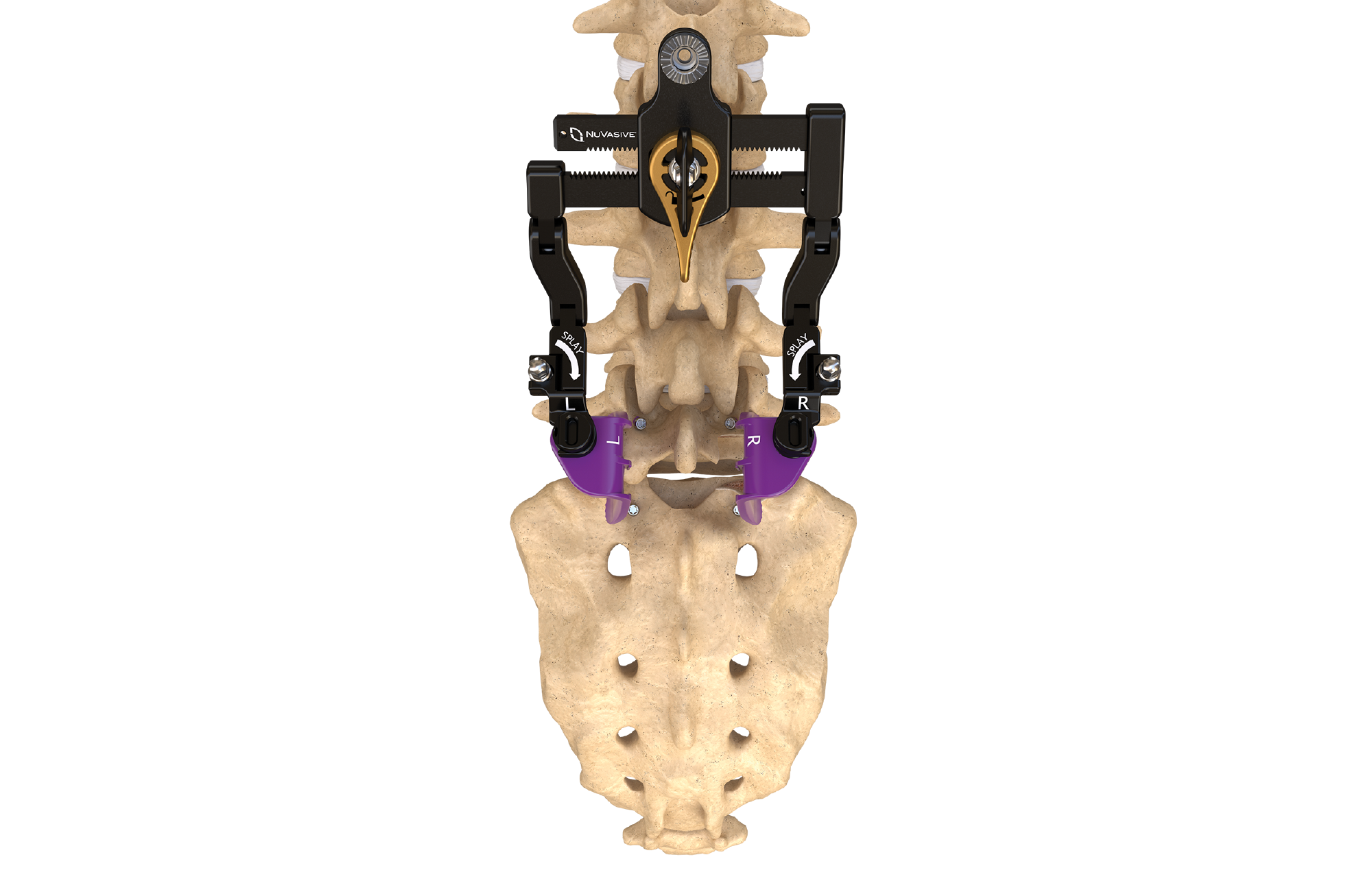
The NuVasive MAS TLIF 2 system offers less invasive, pedicle-based TLIF access and bridges the gap between Open and MIS TLIF approaches. The system features access design that can be adjusted for maximum visibility, functionality, and unimpeded access to known clinical and anatomical landmarks, and integrates with our comprehensive fixation, biologics, and interbody portfolio, featuring a wide range of expandable and next-generation static cages.
The NuVasive MAS Midline system provides unimpeded access and direct visualization of anatomy while minimizing unnecessary tissue and muscle disruption through a familiar midline approach. The system features an access design that can be adjusted to provide decompression, and integrates with our comprehensive fixation, biologics, and interbody implant portfolio, featuring a wide range of expandable and next-generation static cages.
Our posterior expandable interbody portfolio offers multiple footprint options and up to 20° of lordosis to allow surgeons to tailor the implant expansion based on a patient's clinical need.
TLX is designed for anterior column stability and restoration of sagittal alignment with up to 20° of customizable oblique lordosis from a posterior approach.
MLX is designed to be inserted through a conventional TLIF approach, MLX addresses the unique biomechanical loading and lordosis requirements of the lower lumbar spine by expanding to the dimensions of an ALIF graft, helping to optimize stability and maximize endplate coverage.

NuVasive is the first and only company to offer both Porous PEEK and Modulus titanium (Ti) implant offerings for the TLIF procedure. Adhering to the three core principles of AMS, surface, structure, and imaging, NuVasive has pioneered design and manufacturing methods that combine the inherent benefits of porosity with the advantageous material properties of PEEK and Ti.
Cohere TLIF-O is a porous PEEK interbody designed to provide lordosis in the oblique plane to maintain coronal alignment while restoring sagittal balance.
Cohere TLIF-A is a porous PEEK interbody designed for anterior column support for maximized sagittal alignment correction.
Modulus TLIF-O is a porous titanium interbody designed to provide lordosis in the oblique plane to maintain coronal alignment while restoring sagittal balance.
Modulus TLIF-A is a porous titanium interbody designed for anterior column support for maximized sagittal alignment correction.
Reline is the most comprehensive fixation system in spine, offering an easy-to-use yet powerful solution.
Fully integrated with C360, X360 and P360 portfolios, the Reline system offers a complete solution to address a wide range of degenerative pathologies.
Attrax Putty is the first and only synthetic biologic to receive 510(k) clearance for use with thoracolumbar interbody systems1 and is one of only a few bone grafting devices on the market supported by a Level I randomized controlled trial as a bone graft substitute in posterolateral spinal fusions.4
Watch the NuVasive Innovation Event to see our latest innovations.
Connect with our Clinical Professional
Development team to sign up for a course.
From Open TLIFs to Maximum Access Surgery (MAS®) Midline procedures, NuVasive has access, fixation, and interbody solutions to treat the wide spectrum of patient pathologies and surgeon preferences.
The NuVasive Tube System (NTS) is engineered to elevate surgical workflow through efficient, controlled positioning and optimized access. As the MIS access component of our P360 portfolio, NTS provides comprehensive solutions for both TLIF and decompression, furthering our goal to help surgeons deliver patient-specific surgical plans with our pathology-driven solutions, all from the prone position.
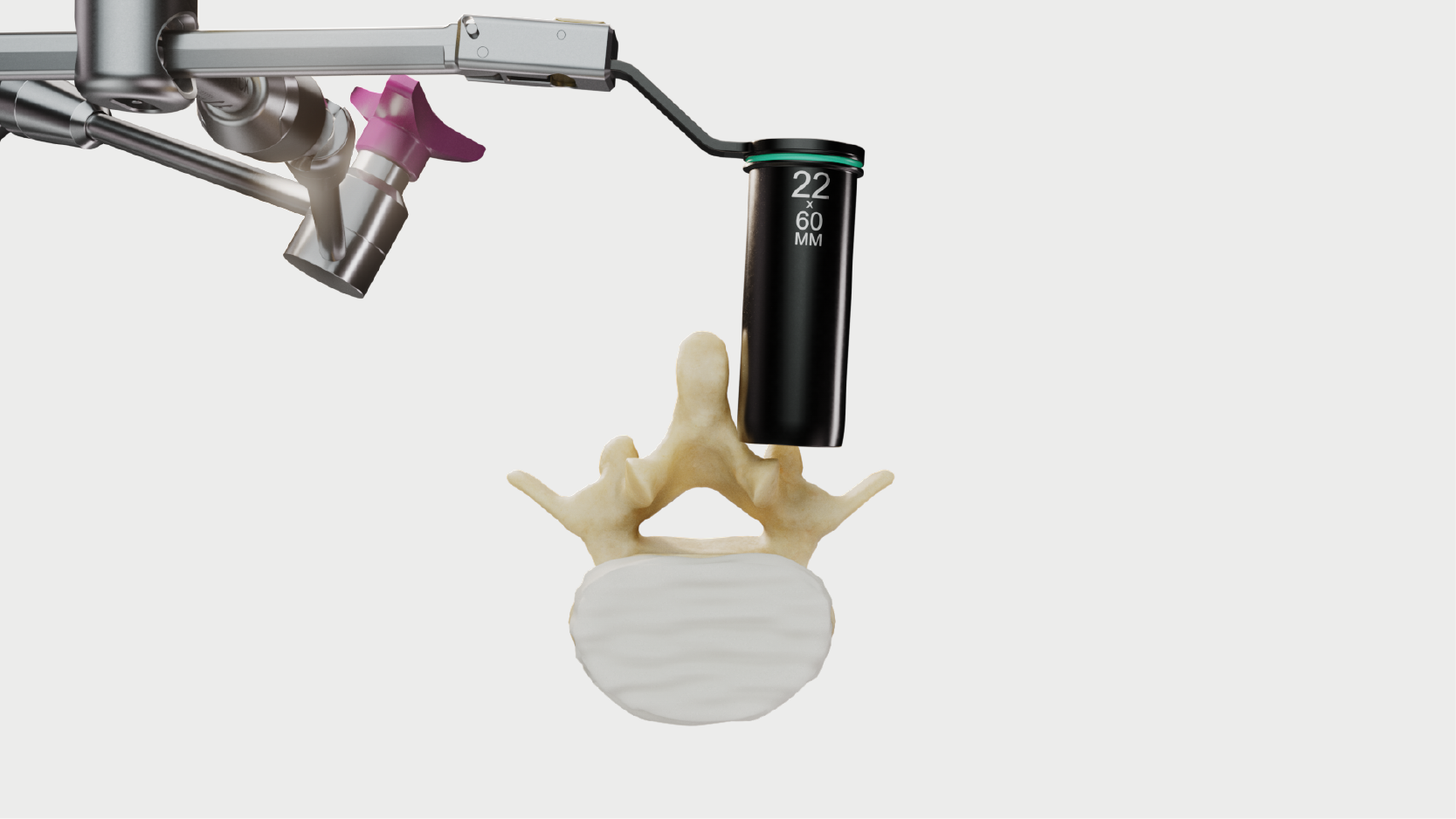
Seamless adjustment.
Increased cranial-caudal angulation and medial-lateral rotation.
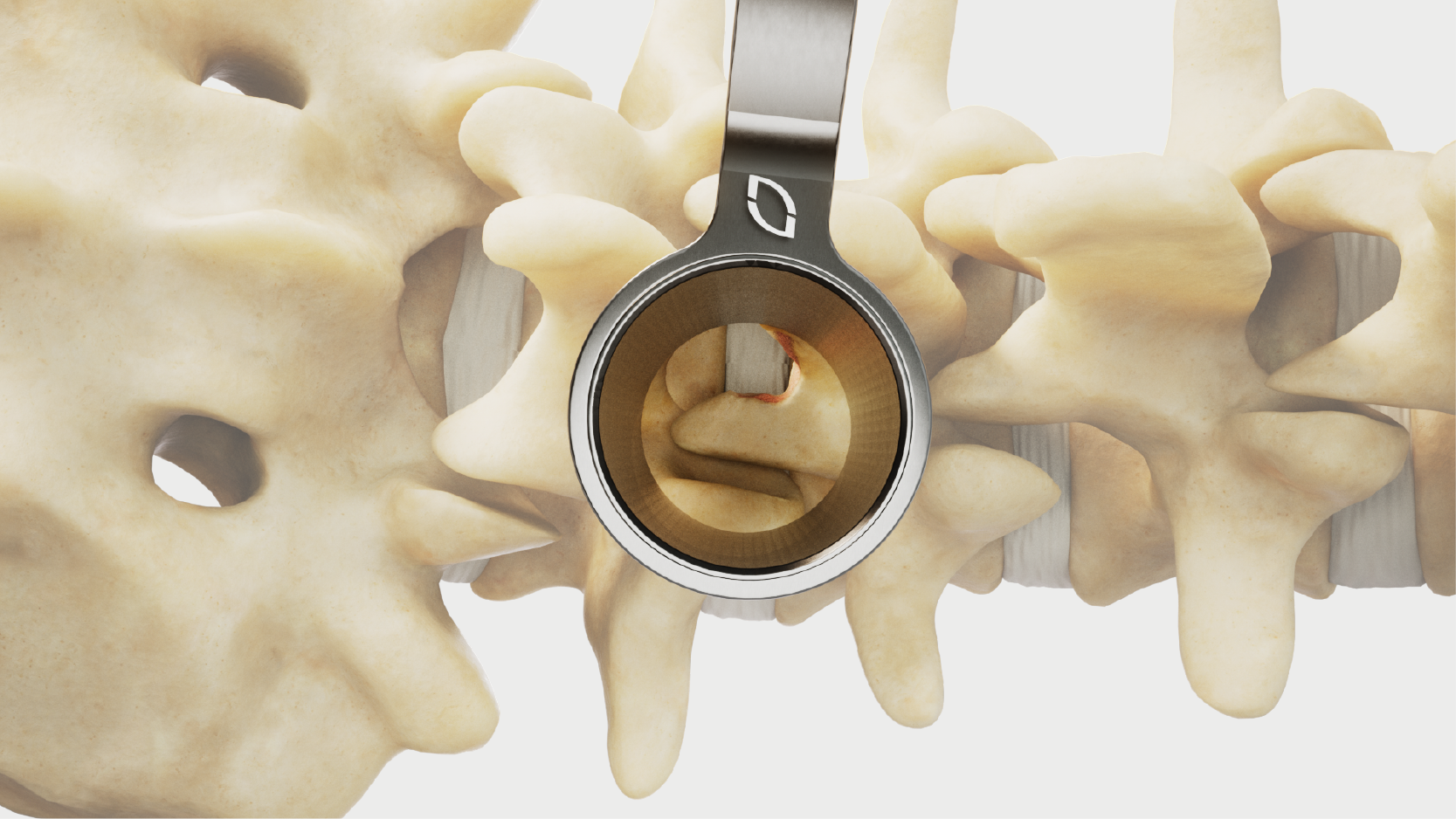
Standard and slotted tube options.
Diameters and lengths to address cervical to lumbar.
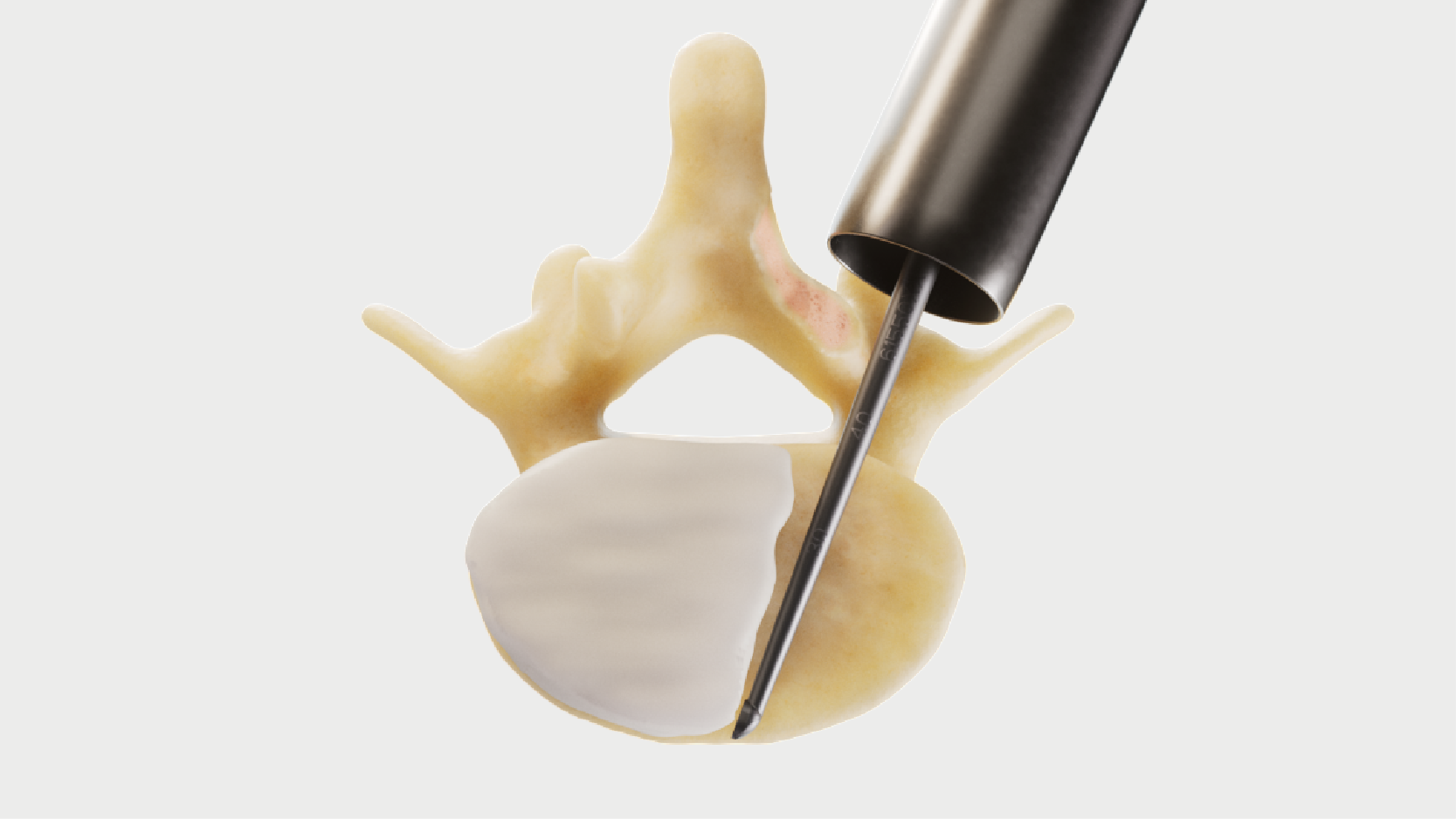
Complementary Micro Decompression instruments.
Thoughtfully designed for superior ergonomics.
The NuVasive MAS TLIF 2 system offers less invasive, pedicle-based TLIF access and bridges the gap between Open and MIS TLIF approaches. The system features access design that can be adjusted for maximum visibility, functionality, and unimpeded access to known clinical and anatomical landmarks, and integrates with our comprehensive fixation, biologics, and interbody portfolio, featuring a wide range of expandable and next-generation static cages.
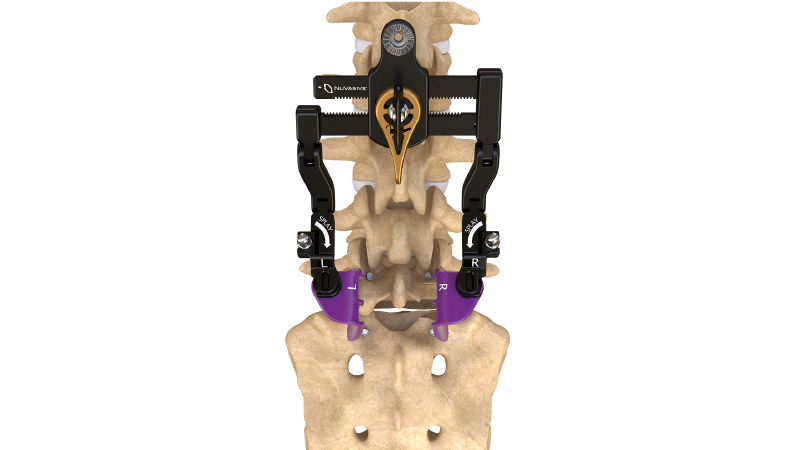
The NuVasive MAS Midline system provides unimpeded access and direct visualization of anatomy while minimizing unnecessary tissue and muscle disruption through a familiar midline approach. The system features an access design that can be adjusted to provide decompression, and integrates with our comprehensive fixation, biologics, and interbody implant portfolio, featuring a wide range of expandable and next-generation static cages.
Our posterior expandable interbody portfolio offers multiple footprint options and up to 20° of lordosis to allow surgeons to tailor the implant expansion based on a patient's clinical need.

TLX is designed for anterior column stability and restoration of sagittal alignment with up to 20° of customizable oblique lordosis from a posterior approach.
MLX is designed to be inserted through a conventional TLIF approach, MLX addresses the unique biomechanical loading and lordosis requirements of the lower lumbar spine by expanding to the dimensions of an ALIF graft, helping to optimize stability and maximize endplate coverage.
NuVasive is the first and only company to offer both Porous PEEK and Modulus titanium (Ti) implant offerings for the TLIF procedure. Adhering to the three core principles of AMS, surface, structure, and imaging, NuVasive has pioneered design and manufacturing methods that combine the inherent benefits of porosity with the advantageous material properties of PEEK and Ti.

Cohere TLIF-O is a porous PEEK interbody designed to provide lordosis in the oblique plane to maintain coronal alignment while restoring sagittal balance.
Cohere TLIF-A is a porous PEEK interbody designed for anterior column support for maximized sagittal alignment correction.

Modulus TLIF-O is a porous titanium interbody designed to provide lordosis in the oblique plane to maintain coronal alignment while restoring sagittal balance.
Modulus TLIF-A is a porous titanium interbody designed for anterior column support for maximized sagittal alignment correction.
Reline is the most comprehensive fixation system in spine, offering an easy-to-use yet powerful solution.
Fully integrated with C360, X360 and P360 portfolios, the Reline system offers a complete solution to address a wide range of degenerative pathologies.
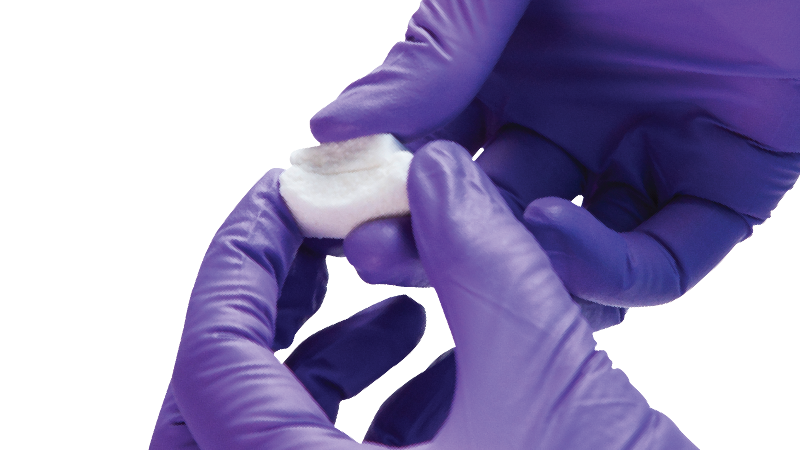
Attrax Putty is the first and only synthetic biologic to receive 510(k) clearance for use with thoracolumbar interbody systems.1
The Attrax ceramic surface has unique microstructure and microporosity that are optimized for bone formation. The unique microarchitecture of Attrax drives the differentiation of mesenchymal stem cells (MSCs) into bone-forming osteoblasts without added growth factors.2 The optimized microarchitecture of Attrax is engineered using tightly controlled parameters for a defined micropore size distribution within 0.3 to 1.1 microns, demonstrated to initiate greater bone formation compared to traditional ceramics.3
Attrax Putty is one of only a few bone grafting devices on the market supported by a Level I randomized controlled trial as a bone graft substitute in posterolateral spinal fusions.4
Watch the NuVasive Innovation Event to see our latest innovations.
Connect with our Clinical Professional Development
team to sign up for a course.
1. As of date of publication, March 2017; data on file.
2.Polini A, Pisignano D, Parodi M, et al. Osteoinduction of human mesenchymal stem cells by bioactive composite sca olds without supplemental osteogenic growth factors. PLoS ONE 2011;6(10):1-8.
3.Duan R, Barbieri D, Luo X, et al. Variation of the bone forming ability with the physicochemical properties of calcium phosphate bone substitutes. Biomater Sci 2018;6:136-45.
4.Lehr MA, Oner CF, Delawi D, et al. E cacy of a standalone microporous ceramic vs. autograft in instrumented posterolateral spinal fusion; a multicenter, randomized, intra-patient controlled, non-inferiority trial. Spine 2020;45(14):944-51.
For important product safety information please visit nuvasive.com/eIFU