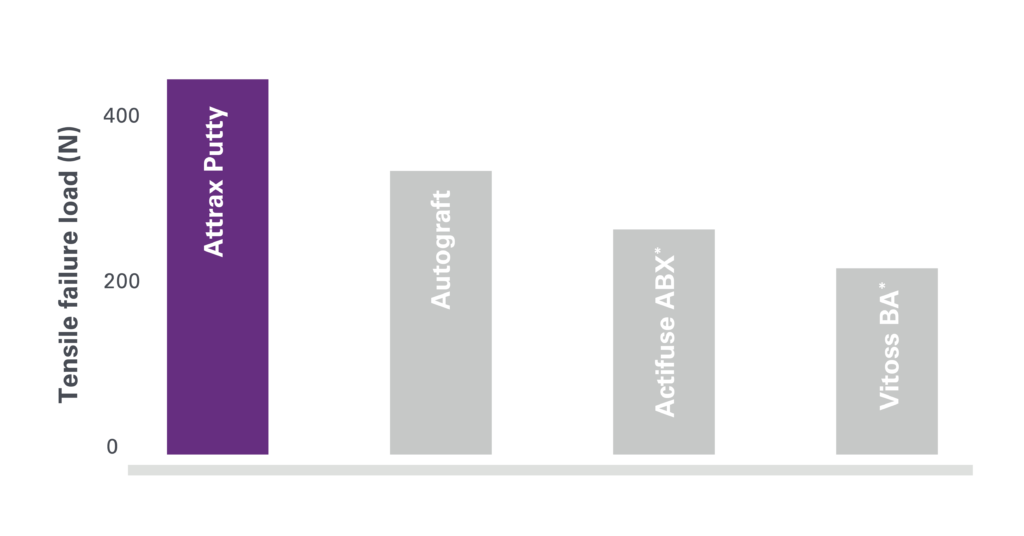
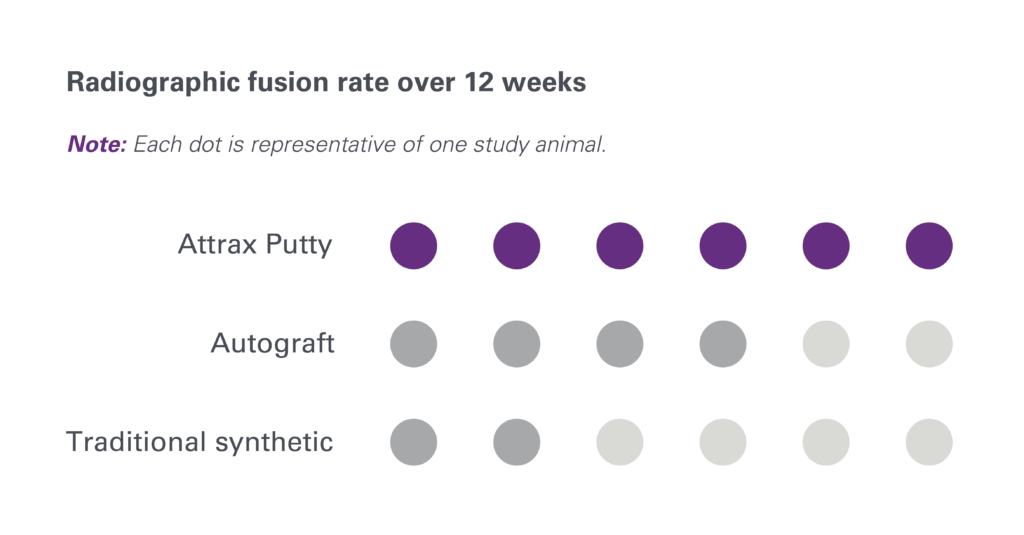
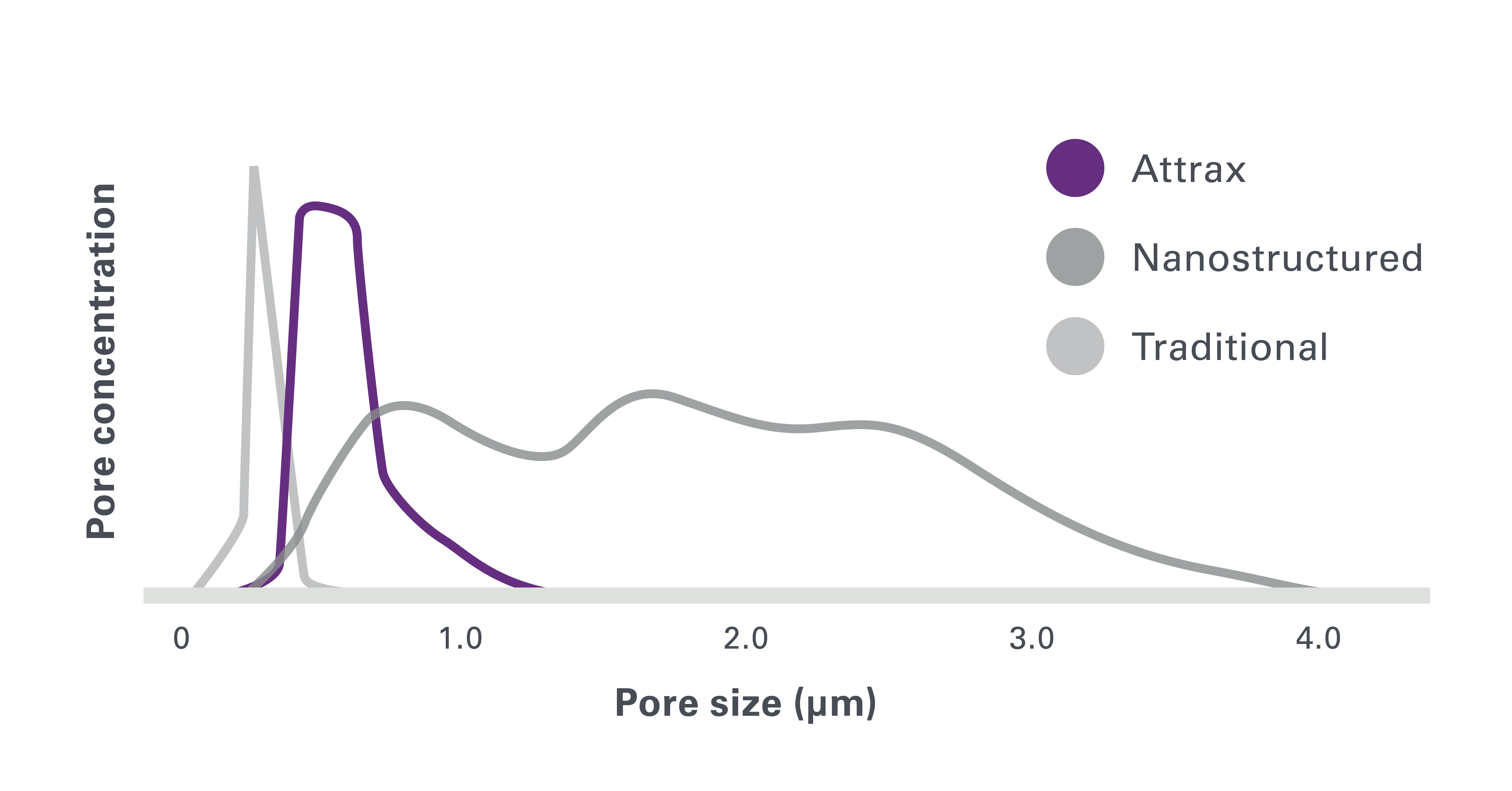
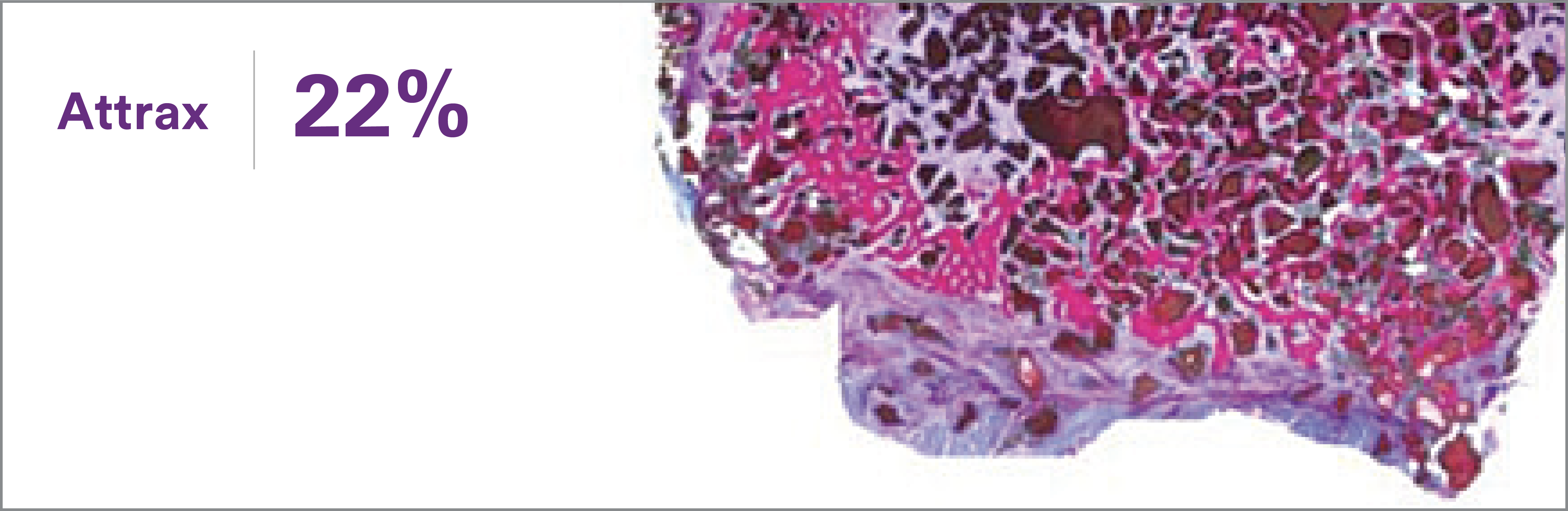
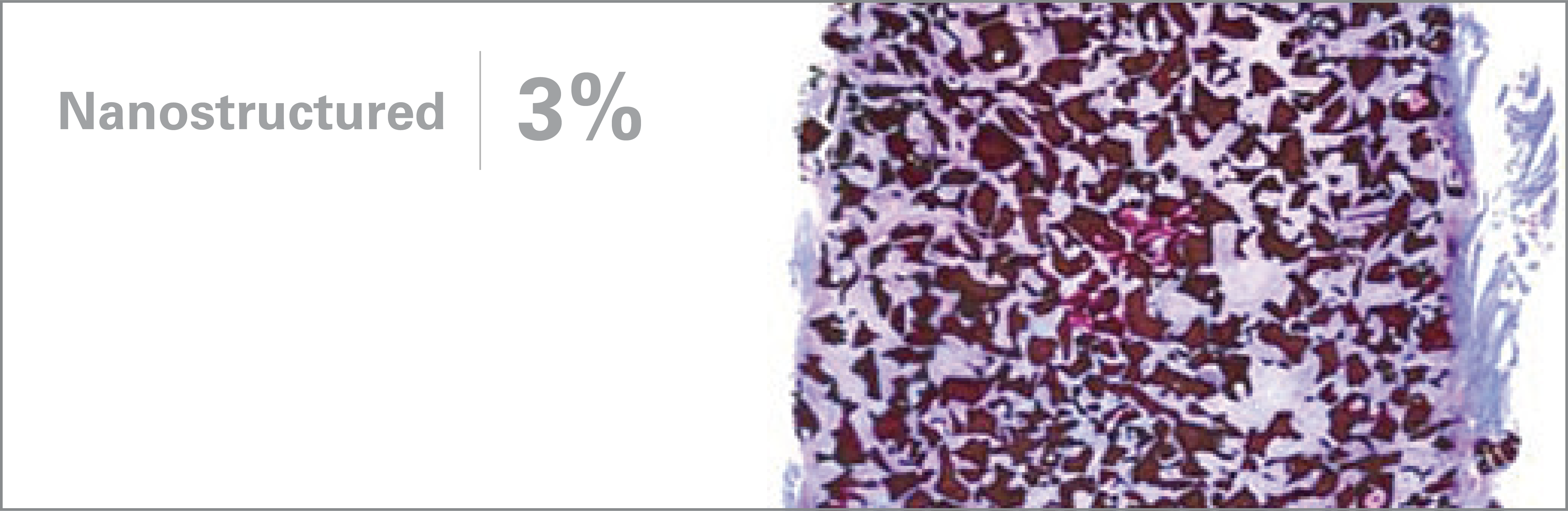
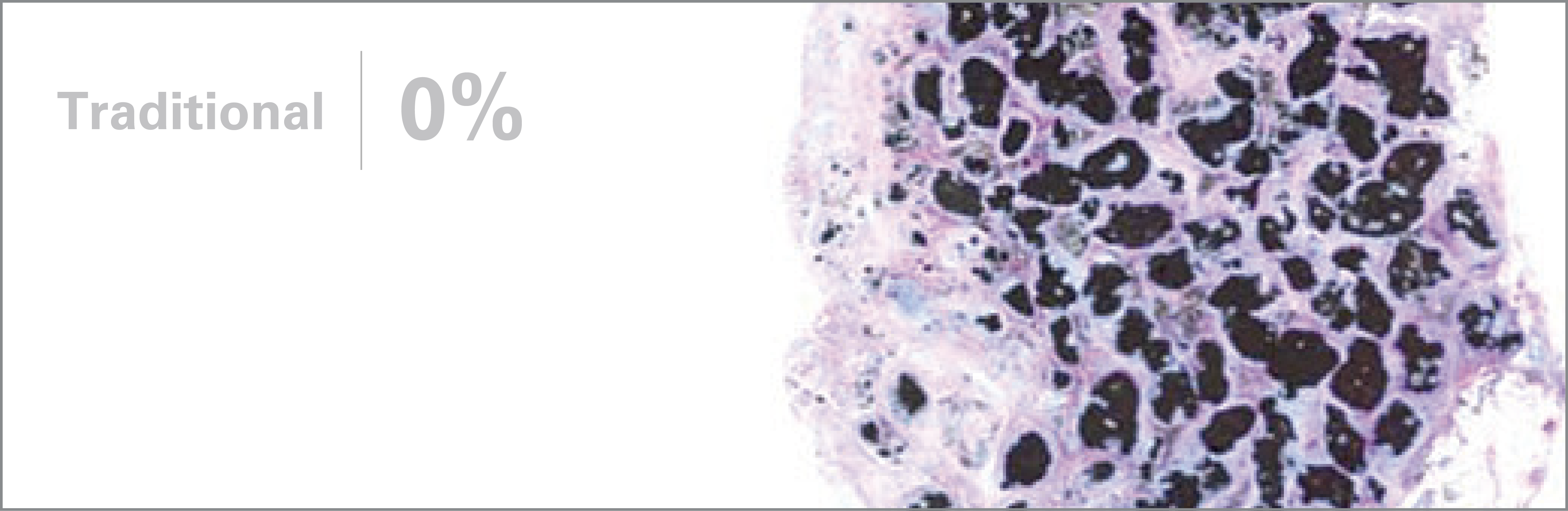
1. Based on review of publicly available materials at the time of this release.
2. Lehr AM, Oner FC, Delawi D, et al. Efficacy of a standalone microporous ceramic versus autograft in instrumented posterolateral spinal fusion. Spine 2020;45(14):944-51.
3. Walsh WR, Degroot F, Bertollo N, et al. Nanostructured TCP in rabbit posterolateral fusion compared to commercial osteobiologics. American Academy of Orthopaedic Surgeons 2011 Annual Meeting. San Diego, CA, USA.
4. Ismailoglu AS, Vizesi F, Cunningham B, et al. Fibrillar collagen/ TCP scaffold in the sheep posterolateral fusion model. Society for Biomaterials Annual Meeting; 2012; New Orleans, LA, USA.
5. Fredericks DC, Smucker JD, Peterson EB, et al. Novel TCP compares favorably to autograft in posterolateral fusion: evaluation in rabbit and sheep models. International Society for the Advancement of Spine Surgery Annual Conference; 2013; Vancouver, BC, Canada.
6. Barbieri D, Yuan H, Ismailoglu AS, et al. Comparison of two moldable calcium phosphate-based bone graft materials in a noninstrumented canine interspinous implantation model. Tissue Eng Part A 2017;23(23- 24):1310-20.
7. Yuan H, Fernandes H, Habibovic P, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. PNAS 2010;107(31):13614-9.