Biologics
The NuVasive Biologics portfolio includes procedurally integrated allogeneic bone graft substitutes and extenders, and synthetic ceramic bone graft substitutes intended to promote bone fusion.
The NuVasive Biologics portfolio includes procedurally integrated allogeneic bone graft substitutes and extenders, and synthetic ceramic bone graft substitutes intended to promote bone fusion.
Fusion is primarily performed to eliminate the pain often caused by abnormal motion of the vertebrae. Fusion is a surgical procedure in which one or more of the vertebrae of the spine are joined together (fused) so that motion no longer occurs between them.
Bone grafting is a surgical technique that involves implanting a bone substitute or extender to help the body achieve spinal fusion. The three essential biologic mechanisms involved in complete bone grafts include osteoconduction (a scaffold for the reparative growth of the natural bone), osteoinduction (signals encouraging undifferentiated cells to become active osteoblasts), and osteogenesis (living bone cells in the graft material contribute to bone remodeling).

Learn how the use of Attrax Putty in Modulus XLIF cages resulted in a 97.8% rate of bone bridging5 from Drs. Eastlack and Mundis as they discuss, “Can a bioactive interbody device reduce the cost burden of achieving lateral lumbar fusion?”, recently published in the Journal of Neurosurgery Spine.
In this video, they review the findings around improved patient outcomes and reduced economic burden when using these products together.
Attrax Putty is now the first and only synthetic biologic to receive 510(k) clearance for use with thoracolumbar interbody systems.
The Attrax ceramic surface has unique microstructure and microporosity that are optimized for bone formation. The unique microarchitecture of Attrax drives the differentiation of mesenchymal stem cells (MSCs) into bone-forming osteoblasts without added growth factors.2 The optimized microarchitecture of Attrax is engineered using tightly controlled parameters for a defined micropore size distribution within 0.3 to 1.1 microns, demonstrated to initiate greater bone formation compared to traditional ceramics.3
Attrax Putty is one of only a few bone grafting devices on the market supported by a Level I randomized controlled trial as a bone graft substitute in posterolateral spinal fusions.4
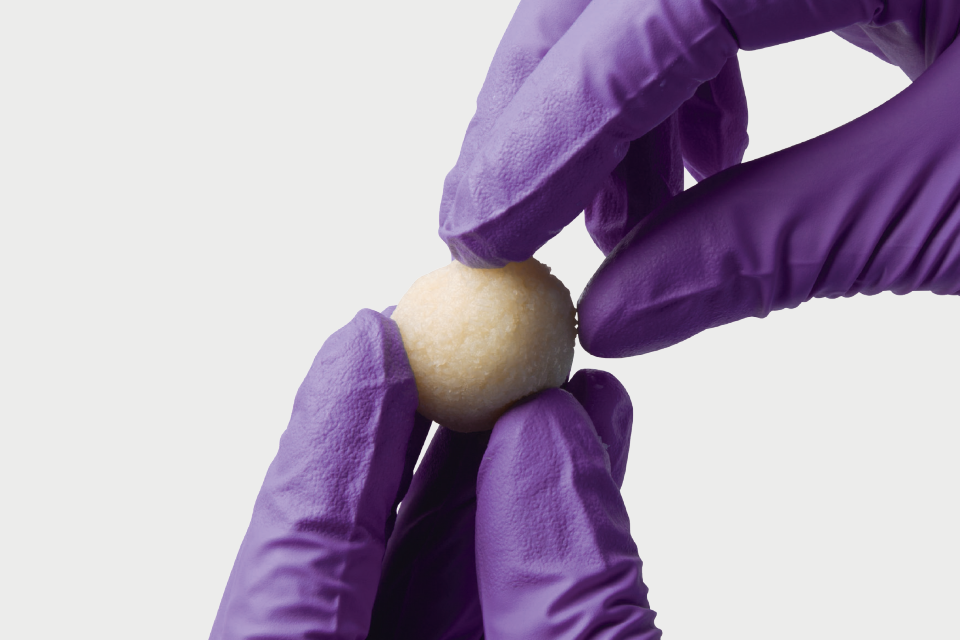
Osteocel is a comprehensive bone graft developed to mimic the properties of a patient's own bone. Osteocel provides a complete and physiologic bone graft that contains all of the necessary components for bone growth: the cells, the signals, and the scaffold.
Osteocel is human tissue donated specifically for human clinical use.
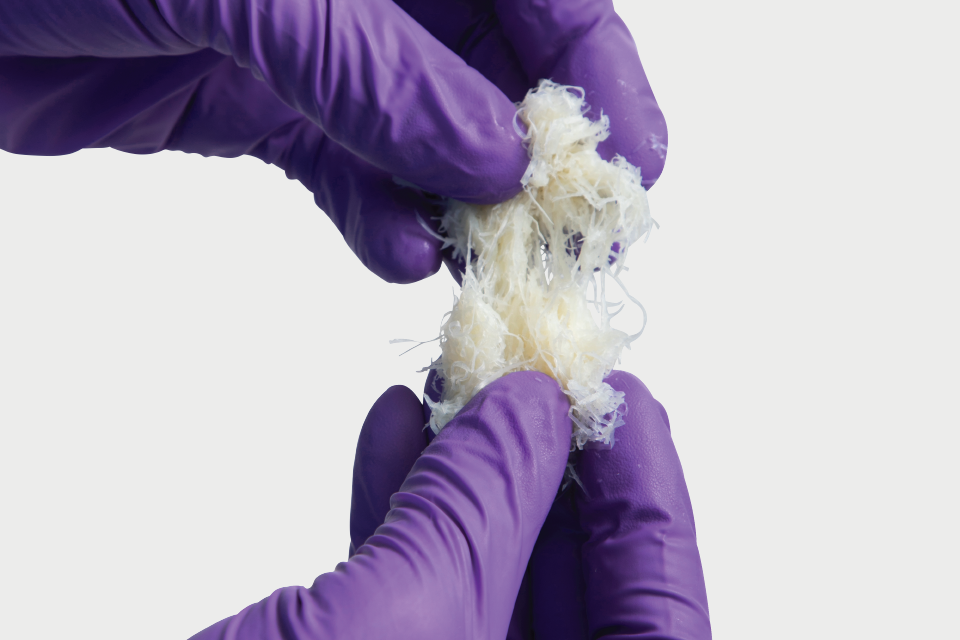
Propel DBM is donated human bone that has been demineralized to remove the minerals and cells contained in the bone while retaining the collagen matrix and growth factors, including bone morphogenetic proteins, inherent to the tissue. Propel DBM Putty and Gel serve as a bone graft extender (extremities, spine, pelvis) and bone void filler of the extremities and pelvis. Propel DBM Putty Plus and Gel Plus contain added cancellous chips and serve as a bone graft extender (extremities, posterolateral spine, pelvis) and bone void filler of the extremities and pelvis. Propel DBM Fibers and Propel DBM Sponge are 100% bone and are intended for homologous use in the repair, replacement, or reconstruction of skeletal defects.
Fusion is primarily performed to eliminate the pain often caused by abnormal motion of the vertebrae. Fusion is a surgical procedure in which one or more of the vertebrae of the spine are joined together (fused) so that motion no longer occurs between them.

Bone grafting is a surgical technique that involves implanting a bone substitute or extender to help the body achieve spinal fusion. The three essential biologic mechanisms involved in complete bone grafts include osteoconduction (a scaffold for the reparative growth of the natural bone), osteoinduction (signals encouraging undifferentiated cells to become active osteoblasts), and osteogenesis (living bone cells in the graft material contribute to bone remodeling).
Learn how the use of Attrax Putty in Modulus XLIF cages resulted in a 97.8% rate of bone bridging5 from Drs. Eastlack and Mundis as they discuss, “Can a bioactive interbody device reduce the cost burden of achieving lateral lumbar fusion?”, recently published in the Journal of Neurosurgery Spine.
In this video, they review the findings around improved patient outcomes and reduced economic burden when using these products together.






The Attrax ceramic surface has unique microstructure and microporosity that are optimized for bone formation. The unique microarchitecture of Attrax drives the differentiation of mesenchymal stem cells (MSCs) into bone-forming osteoblasts without added growth factors.2 The optimized microarchitecture of Attrax is engineered using tightly controlled parameters for a defined micropore size distribution within 0.3 to 1.1 microns, demonstrated to initiate greater bone formation compared to traditional ceramics.3
Attrax Putty is one of only a few bone grafting devices on the market supported by a Level I randomized controlled trial as a bone graft substitute in posterolateral spinal fusions.4
Attrax Scaffold
Attrax Putty
Propel DBM is donated human bone that has been demineralized to remove the minerals and cells contained in the bone while retaining the collagen matrix and growth factors, including bone morphogenetic proteins, inherent to the tissue. Propel DBM Putty and Gel serve as a bone graft extender (extremities, spine, pelvis) and bone void filler of the extremities and pelvis. Propel DBM Putty Plus and Gel Plus contain added cancellous chips and serve as a bone graft extender (extremities, posterolateral spine, pelvis) and bone void filler of the extremities and pelvis. Propel DBM Fibers and Propel DBM Sponge are 100% bone and are intended for homologous use in the repair, replacement, or reconstruction of skeletal defects.
Propel DBM Putty and Gel
Propel DBM Putty Plus and Gel Plus
Propel DBM Fibers
Probel DBM Sponge

1. As of date of publication, March 2017; data on file.
2. Polini A, Pisignano D, Parodi M, et al. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE 2011;6(10):1-8.
3. Duan R, Barbieri D, Luo X, et al. Variation of the bone forming ability with the physicochemical properties of calcium phosphate bone substitutes. Biomater Sci 2018;6:136-45.
4. Lehr MA, Oner CF, Delawi D, et al. Efficacy of a standalone microporous ceramic vs. autograft in instrumented posterolateral spinal fusion; a multicenter, randomized, intra-patient controlled, non-inferiority trial. Spine 2020;45(14):944-51.
5. Malone, H., Mundis, G. M., Jr., Collier, M, et al. Can a bioactive interbody device reduce the cost burden of achieving lateral lumbar fusion? Journal of Neurosurgery Spine. Published online June 3, 2022; DOI: 10.3171/2022.4.SPINE211070