Attrax
Attrax is a synthetic bone void filler designed to drive bone formation with a uniquely engineered bioactive and osteoconductive surface structure.
Supported by Level I data
In the only Level I randomized controlled trial of a synthetic bone graft substitute in instrumented PLF, Attrax Putty demonstrated non-inferiority to autograft1
- Randomized, intrapatient controlled trial of 100 patients across four different hospitals
- Single or multi-level (T10–S1) instrumented PLF, deformity, instability and/or expected instability
- Fusion rate at 1 year by CT, each level and each side scored by blinded observers
Peer-to-peer discussion between Drs. Robert Eastlack and Gregory Mundis
Learn how the use of Attrax Putty in Modulus XLIF cages resulted in a 97.8% rate of bone bridging2 from Drs. Eastlack and Mundis as they discuss, “Can a bioactive interbody device reduce the cost burden of achieving lateral lumbar fusion?”, published in the Journal of Neurosurgery Spine.
In this video, Drs. Eastlack and Mundis discuss how they achieved improved patient outcomes while reducing the economic burden to their health system when using Attrax Putty with Modulus XLIF.
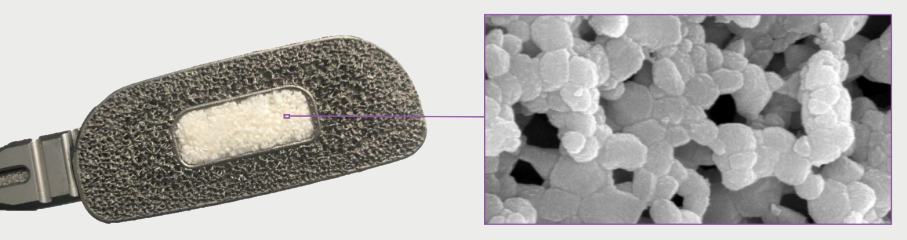
Unique surface optimized architecture elicits bone formation without added cells or growth factors
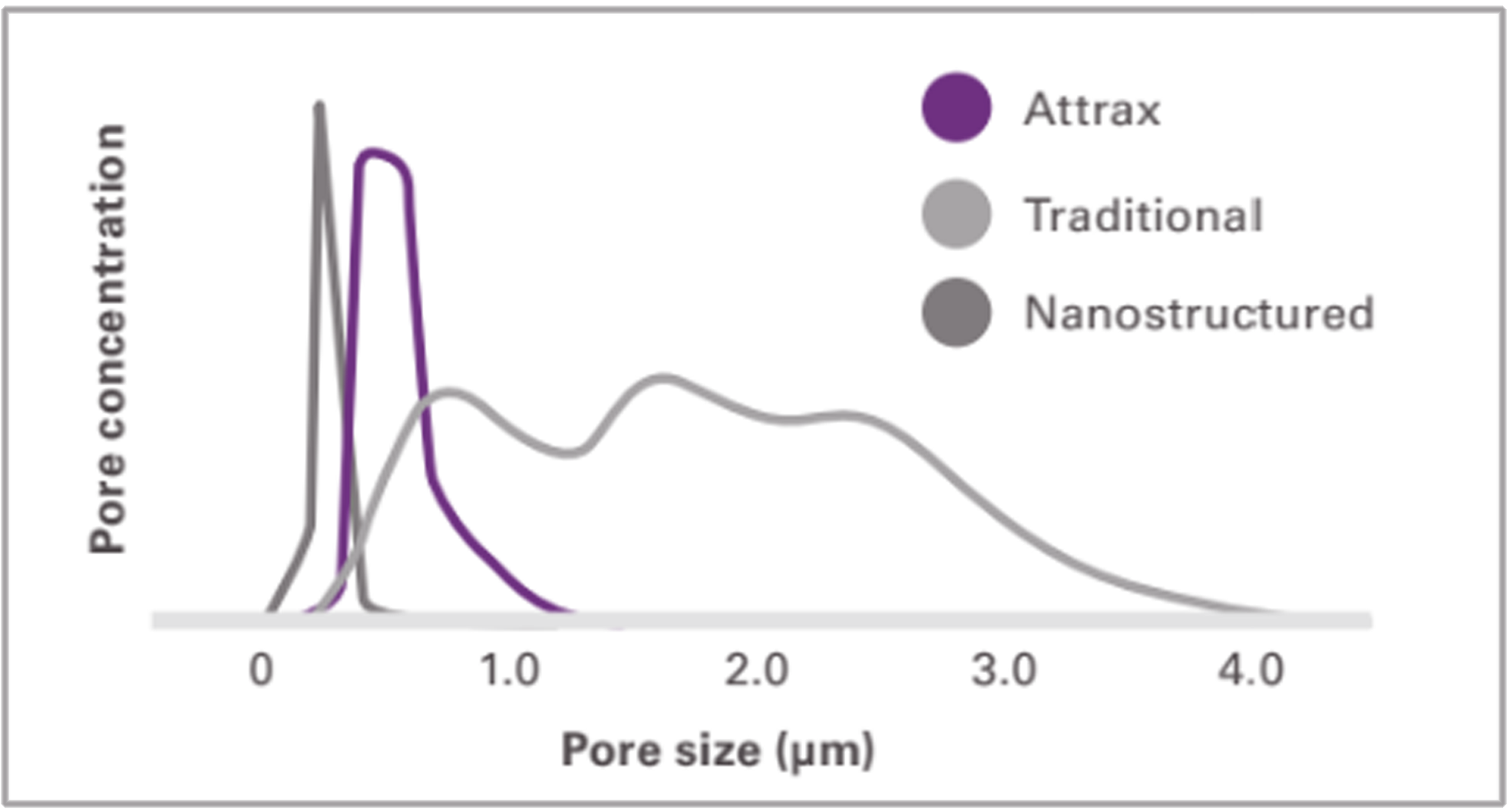
The optimized microarchitecture of Attrax is engineered using tightly controlled parameters for a defined micropore size distribution within 0.3 to 1.1 microns.
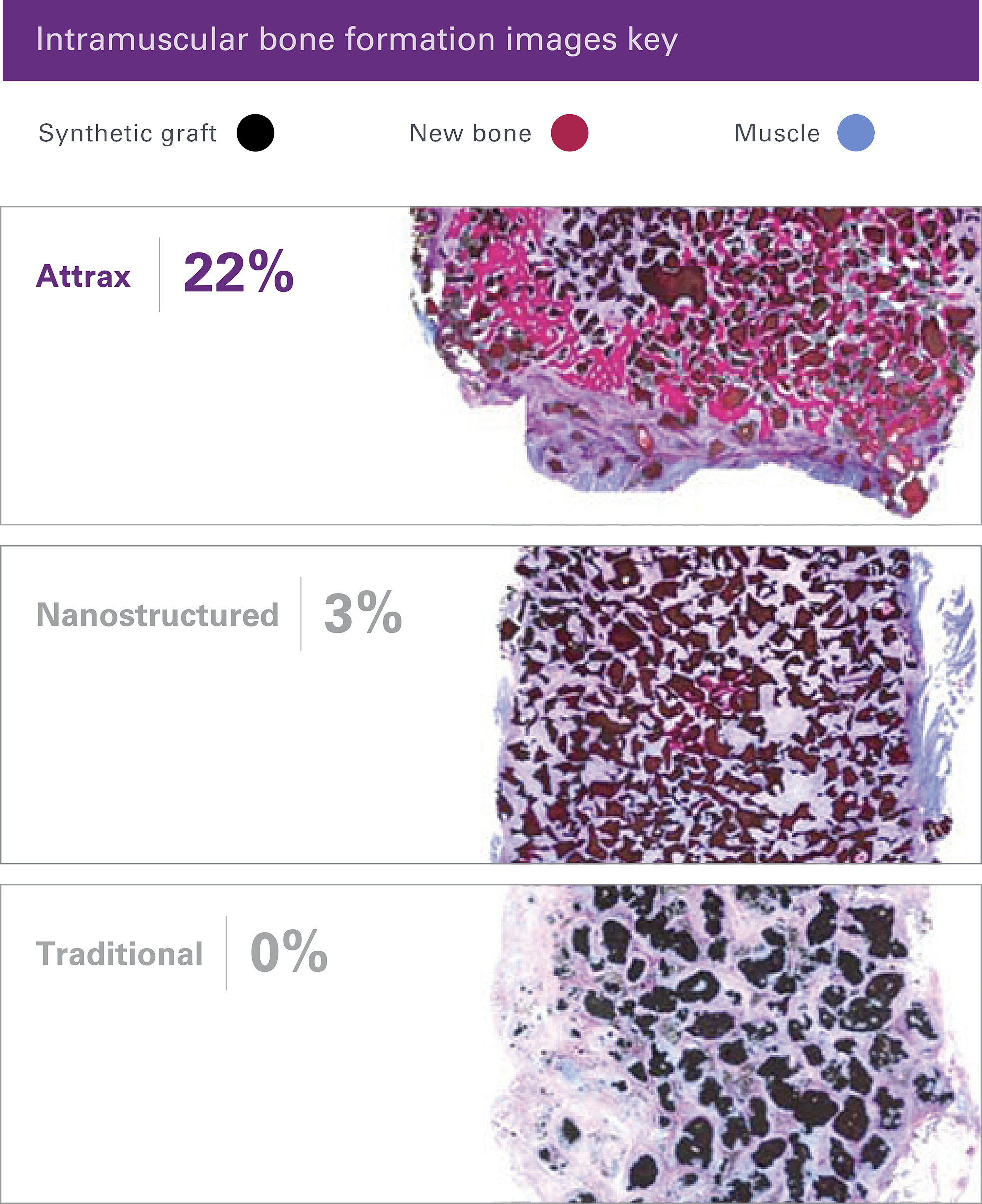
The unique microarchitecture of Attrax drives the differentiation of mesenchymal stem cells (MSCs) into bone-forming osteoblasts without added growth factors.3
Traditional calcium phosphate materials falling outside of this specification do not possess the unique ability of Attrax to form bone consistently in intramuscular defects.
High-performance handling
Best-in-class carriers provide for moldable and cohesive handling properties in a variety of procedurally designed shapes and sizes.
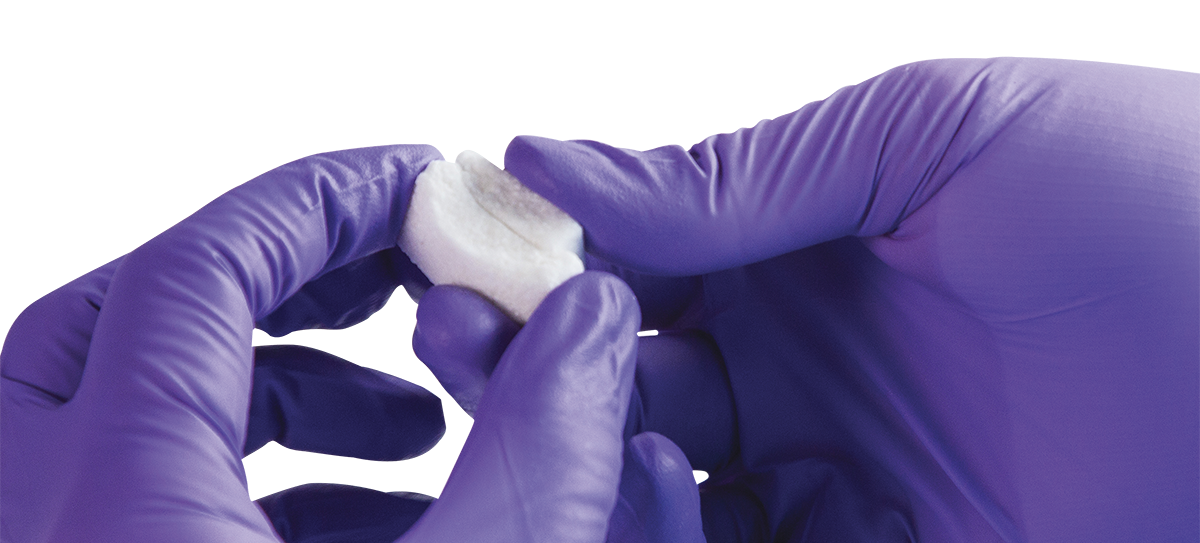
Attrax Putty
Alkylene oxide copolymer carrier
Selected for its ability to be molded into a variety of shapes for intraoperative flexibility
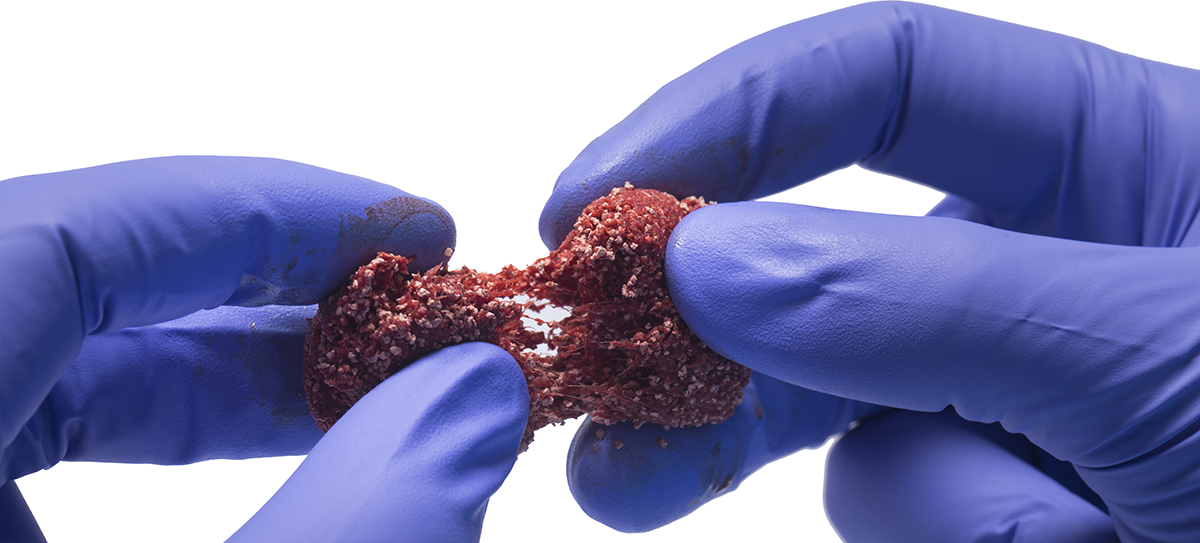
Attrax Scaffold
Entangled collagen matrix
Absorbs biological fluids (e.g., bone marrow aspriate)
Provides compression resistance
Modulus Cervical receives expanded indications from the FDA
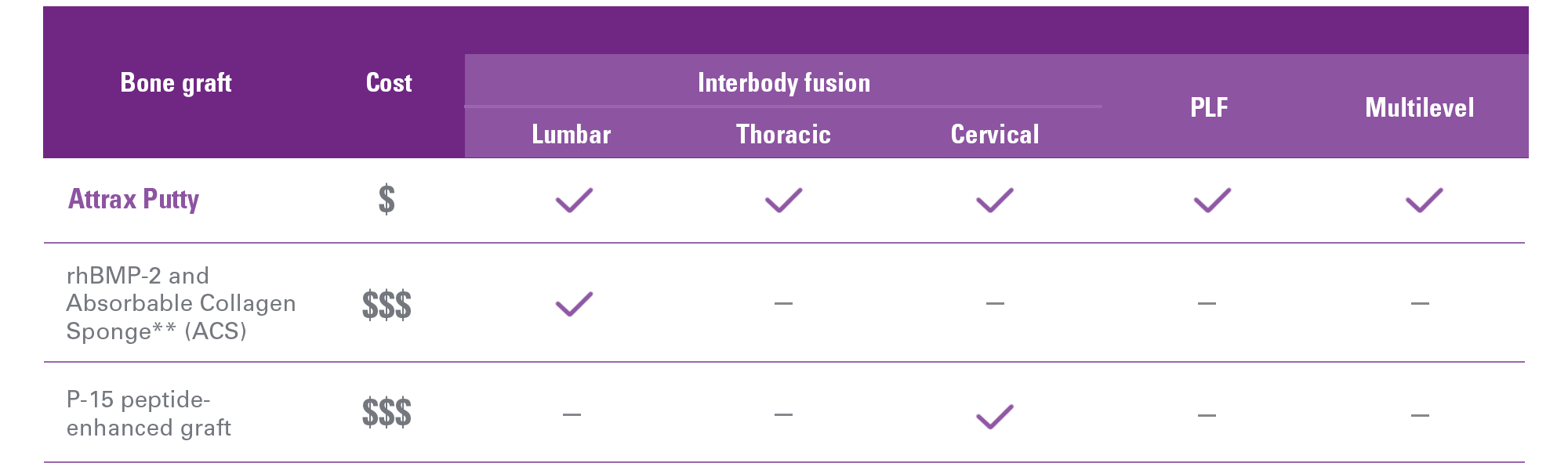
Bone void fillers are indicated to be used in cervical and thoracolumbar interbody fusion devices
Modulus Cervical has received "510(k)" clearance from the FDA that specifies use with a bone void filler in the cervical spine. Now the complete Modulus static cage portfolio can be used with bone void fillers (e.g., Attrax Putty), covering all cervical and thoracolumbar applications.
On-label indications
Attrax Putty may offer a cost effective solution across a broad spectrum of spinal fusion procedures.

Learn more about Attrax
Attrax
Attrax is a synthetic bone void filler designed to drive bone formation with a uniquely engineered bioactive and osteoconductive surface structure.
Supported by Level I data
In the only Level I randomized controlled trial of a synthetic bone graft substitute in instrumented PLF, Attrax Putty demonstrated non-inferiority to autograft1
Randomized, intrapatient controlled trial of 100 patients across four different hospitals
Single or multi-level (T10–S1) instrumented PLF, deformity, instability and/or expected instability
Fusion rate at 1 year by CT, each level and each side scored by blinded observers
Peer-to-peer discussion between Drs. Robert Eastlack and Gregory Mundis
Learn how the use of Attrax Putty in Modulus XLIF cages resulted in a 97.8% rate of bone bridging2 from Drs. Eastlack and Mundis as they discuss, “Can a bioactive interbody device reduce the cost burden of achieving lateral lumbar fusion?”, published in the Journal of Neurosurgery Spine.
In this video, Drs. Eastlack and Mundis discuss how they achieved improved patient outcomes while reducing the economic burden to their health system when using Attrax Putty with Modulus XLIF.
Unique surface optimized architecture elicits bone formation without added cells or growth factors
The optimized microarchitecture of Attrax is engineered using tightly controlled parameters for a defined micropore size distribution within 0.3 to 1.1 microns.

The unique microarchitecture of Attrax drives the differentiation of mesenchymal stem cells (MSCs) into bone-forming osteoblasts without added growth factors.3
Traditional calcium phosphate materials falling outside of this specification do not possess the unique ability of Attrax to form bone consistently in intramuscular defects.

High-performance handling
Best-in-class carriers provide for moldable and cohesive handling properties in a variety of procedurally designed shapes and sizes.

Attrax Putty
Alkylene oxide copolymer carrier
Selected for its ability to be molded into a variety of shapes for intraoperative flexibility

Attrax Scaffold
Entangled collagen matrix
Absorbs biological fluids (e.g., bone marrow aspriate)
Provides compression resistance
Modulus Cervical receives expanded indications from the FDA

Bone void fillers are indicated to be used in cervical and thoracolumbar interbody fusion devices
Modulus Cervical has received "510(k)" clearance from the FDA that specifies use with a bone void filler in the cervical spine. Now the complete Modulus static cage portfolio can be used with bone void fillers (e.g., Attrax Putty), covering all cervical and thoracolumbar applications.
On-label indications
Attrax Putty may offer a cost effective solution across a broad spectrum of spinal fusion procedures.


Learn more about Attrax
1. Lehr MA, Oner CF, Delawi D, et al. Efficacy of a standalone microporous ceramic vs. autograft in instrumented posterolateral spinal fusion; a multicenter, randomized, intra-patient controlled, non-inferiority trial. Spine 2020;45(14):944-51.
2. Malone, H., Mundis, G. M., Jr., Collier, M, et al. Can a bioactive interbody device reduce the cost burden of achieving lateral lumbar fusion? J Neurosurg Spine. Published online ahead of print June 3, 2022; DOI: 10.3171/2022.4.SPINE211070
3. Yuan H, Fernandes H, Habibovic P, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci USA 2010;107(31):13614-9.