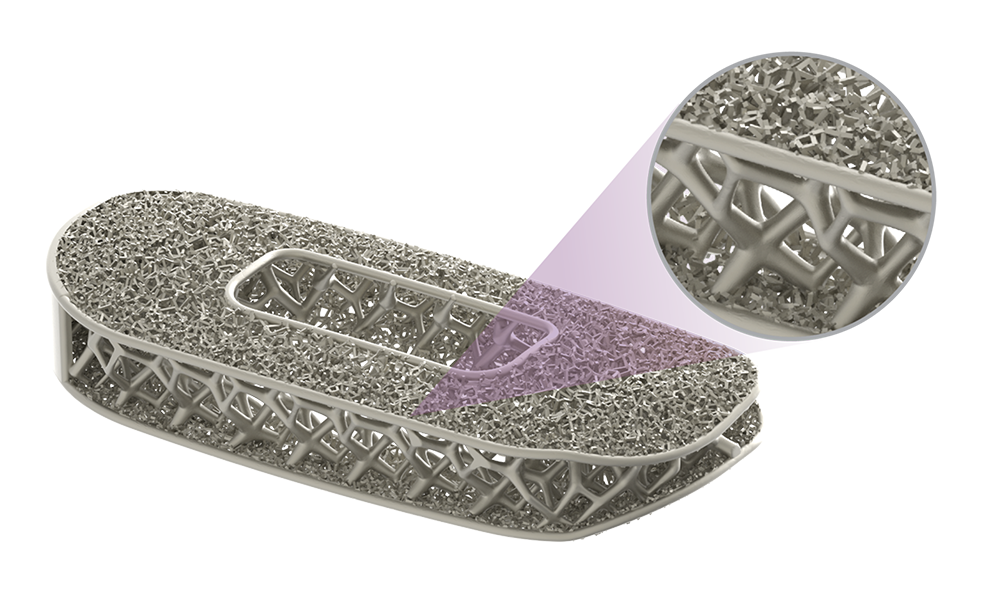
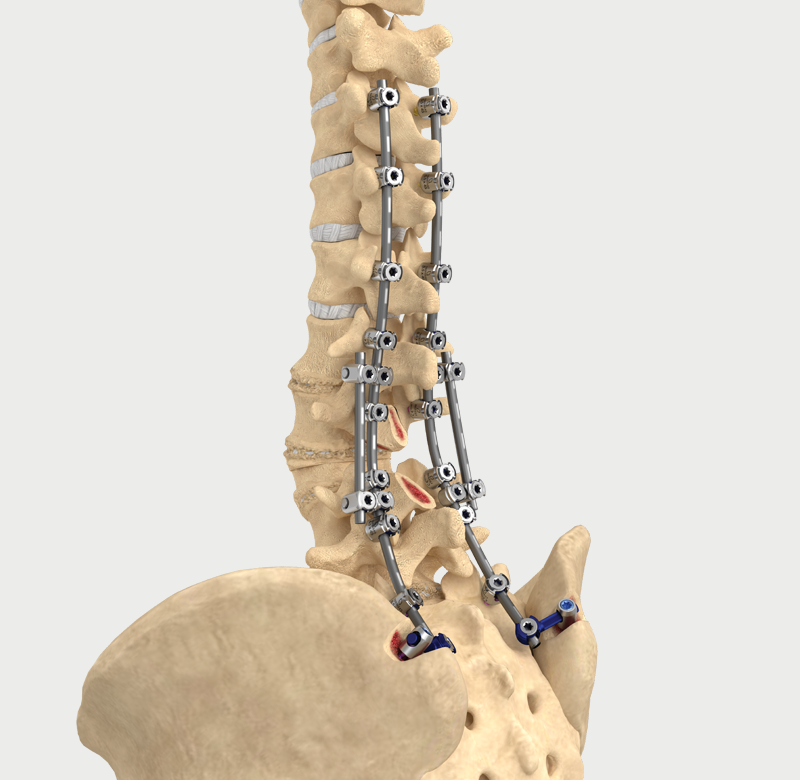
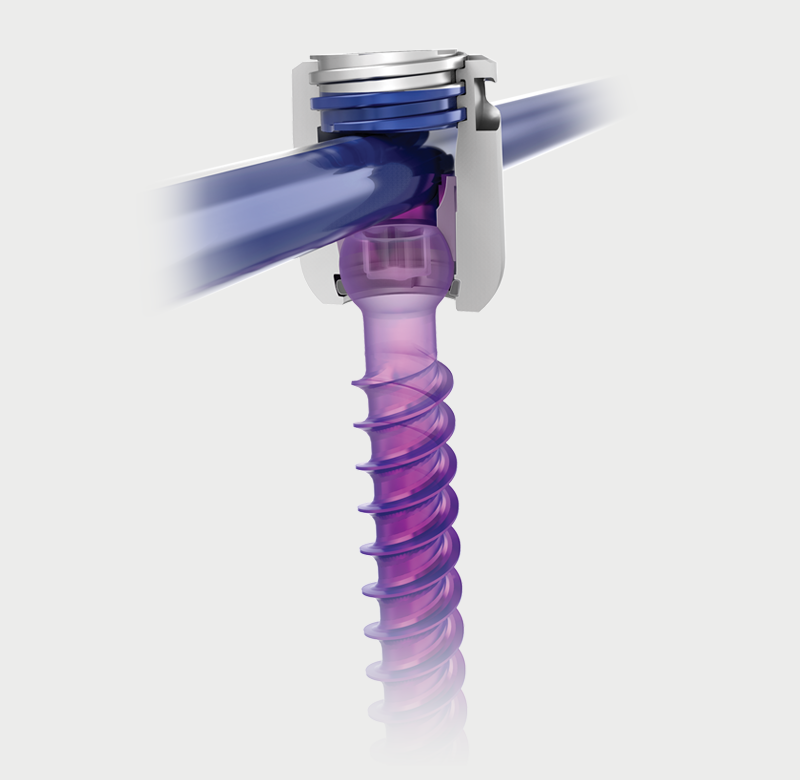
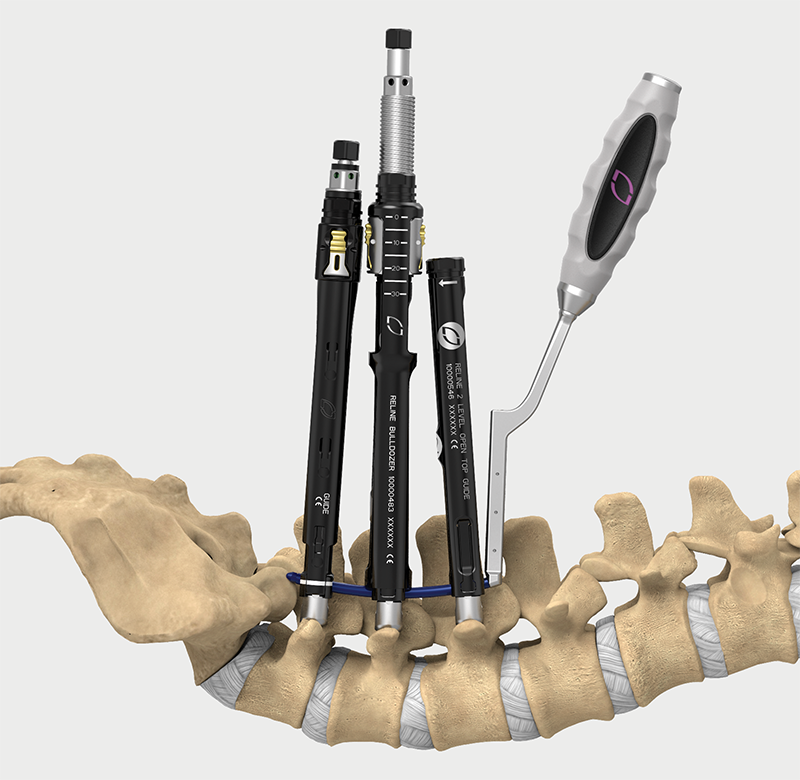
Helical Flange is a registered trademark of Roger P. Jackson.
Simplify Disc CE mark is for one level only.
Not all products referenced may be available in your market. For region-specific product offerings, contact your NuVasive representative or visit nuvasive.com/Contact
For important product safety information, visit nuvasive.com/eIFU
1. Drazin D, Kim TT, Johnson JP. Simultaneous lateral interbody fusion and posterior percutaneous instrumentation: early experience and technical considerations. Biomed Res Int 2015:Article ID 458284.
2. Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg 2003;98(2):149-55.24.
3. Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am 2008;90(1):62-9.
4. Lucio JC, VanConia RB, DeLuzio KJ, et al. Economics of less invasive spinal surgery: an analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag Healthc Policy 2012;5:65-74.
5. Preclinical data on file. Data may not be representative of clinical results. TR 96047.
6. Chatham LS, Patel VV, Yakacki CM, et al. Interbody spacer material properties and design conformity for reducing subsidence during lumbar interbody fusion. J Biomech Eng 2017;139(5):051005.