
This year at the North American Spine Society’s Annual Meeting (NASS), NuVasive showcased our industry leading procedurally integrated technologies to transform spine surgery. NASS is our largest tradeshow of the year and an opportunity to demonstrate our commitment to changing lives through best-in-class technology with surgeons from around the globe. For those of you that missed NASS, we’ll highlight the five key takeaways:

1. Private evening event unveiling Pulse Robotics.The event took place at the Museum of Contemporary Art Chicago to unveil Pulse Robotics, an application that has been designed to allow surgeons to perform robotic-assisted spine surgery with the Pulse platform. With hundreds in attendance, CEO Chris Barry and President Matt Link addressed the guests about NuVasive’s commitment to provide surgeons with enabling technologies like Pulse Robotics that help improve the standard of spine care through less invasive surgery.

2. Jerome Bettis at NASS. NuVasive Ambassador and XLIF patient, Jerome Bettis, made a special appearance at NASS. Jerome spoke with local media—Score 670 radio and NCS Sports Chicago—about his journey with back pain and how NuVasive changed his life. He also attended the Pulse Robotics unveiling event where he shared the stage with President Matt Link, and his surgeon, Dr. Juan Uribe. Jerome shared about the importance of selecting the right surgeon and the right procedure, and how XLIF has transformed his life.
3. MIS Lab with Dr. Juan Uribe. NuVasive hosted a Minimally Invasive Transpsoas Lateral Interbody Fusion lab during the general session with Dr. Juan Uribe performing the procedure and Dr. Thomas Mroz moderating the presentation. Dr. Uribe spoke to the clinically differentiated advantages of the XLIF procedure, and demonstrated the industry’s leading lateral solution live in the cadaveric lab.
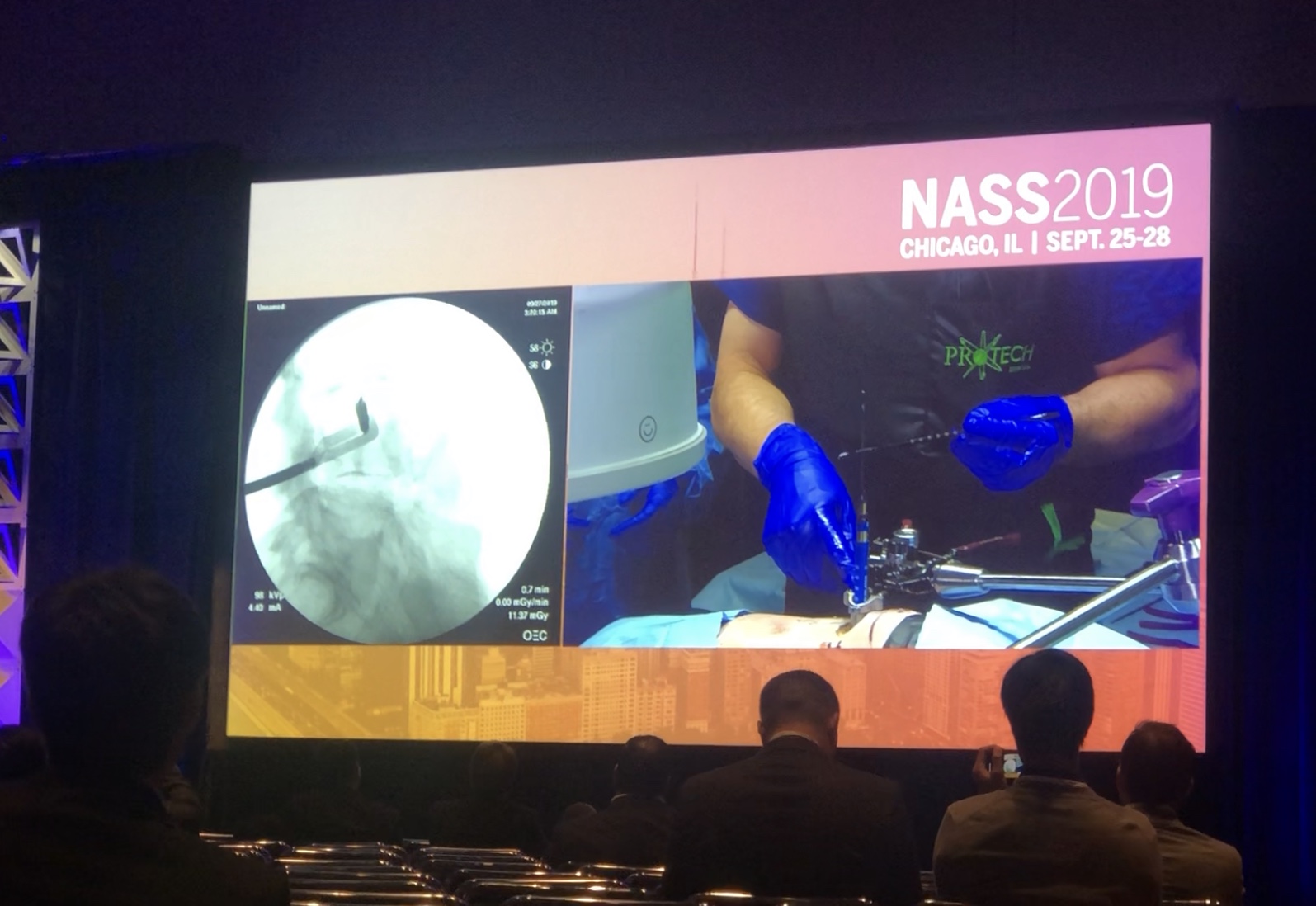
4. Surgeon demonstrations of the X360 system and Pulse platform. We had multiple X360 and Pulse booth demonstrations for NASS attendees throughout the week. Multiple surgeon partners presented with NuVasive marketing and R&D teams on how the integrated technologies will change the future of the OR. The X360 system powered by Surgical Intelligence™, includes XLIF®, XALIF™, XFixation™ and the Pulse platform. The integration of these procedures into a single system allows for significant time savings through the use of modern, less invasive techniques performed with the patient in lateral throughout the entire surgery. The Pulse platform is an integrated technology system designed to improve a surgeon’s ability to utilize minimally invasive surgical techniques, which have been shown to reduce blood loss, hospital stays and result in less operative morbidity compared to open spine surgery. Together, X360 and Pulse will integrate multiple enabling technologies to improve workflow, reduce variability, and increase the reproducibility of surgical outcomes.
5. Women in Spine Commercial Listening Tour. Women in Spine (WIS), our first employee resource group, is a women-led organization that is focused on strengthening NuVasive by highlighting the value of diversity, inclusion and engagement while providing professional development opportunities for all Shareowners. Since its inception this year, WIS has been traveling across the globe on a listening tour, making stops to meet Shareowners across NuVasive locations. These listening tours are a great opportunity to intake the resources that Shareowners need in order to grow and succeed as well as network cross-functionally within the organization. During NASS, WIS gathered commercial team members, neurophysiologists, and other Shareowners from the surrounding area for networking and a short presentation by CEO Chris Barry and Europe Leader Erin McEachren, as well as guest speaker, Dr. Corinna Zygourakis, Stanford Health Care neurosurgeon.
Thank you to the surgeons, Jerome Bettis, and all the Shareowners that helped make NASS 2019 a success!
* Certain applications of the Pulse platform are under development and not available for commercial sale; robotics and smart tools are not cleared for use by the FDA.