Hill & Strenge: Early clinical outcomes comparing porous peek, smooth peek, and structural allograft interbody devices for anterior cervical discectomy and fusion

Background:
While most studies place an emphasis on radiographic fusion rates, those results do not all necessarily show an improvement in functional clinical outcomes in patients. The objective of this single-site retrospective study was to comparatively evaluate early clinical efficacy in patients undergoing ACDF with porous PEEK interbody implants versus structural allograft or traditional smooth PEEK implants.
Method:
In this study, 167 consecutive patients presenting with cervical degenerative disc disease and radiculopathy underwent an ACDF treated with either porous PEEK Cohere, smooth PEEK, or structural allograft implants. Clinical outcomes (i.e. NDI, modified Oswestry, and VAS) were collected from two weeks to 12 months postoperatively.
Fixed variables included:
- the same standard ACDF procedure,
- the same instrumentation for anterior fixation, and
- the same cellular allograft material to fill the graft aperture prior to implantation.
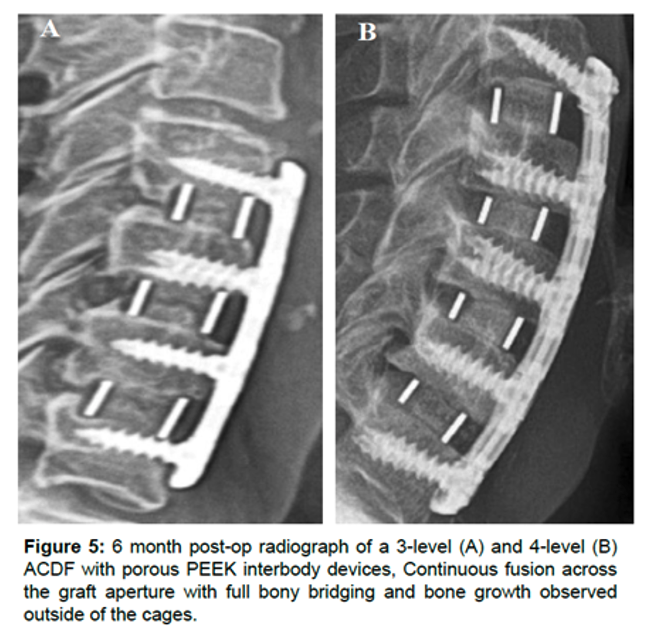
Results:
Patients treated with porous PEEK Cohere implants were associated with significantly greater improvements in NDI and pain score improvements as early as six weeks and sustained through 12 months vs. smooth PEEK and structural allograft.
24 month or later follow-up data are required to determine the effect on the different interbody materials on longer-term clinical outcomes.
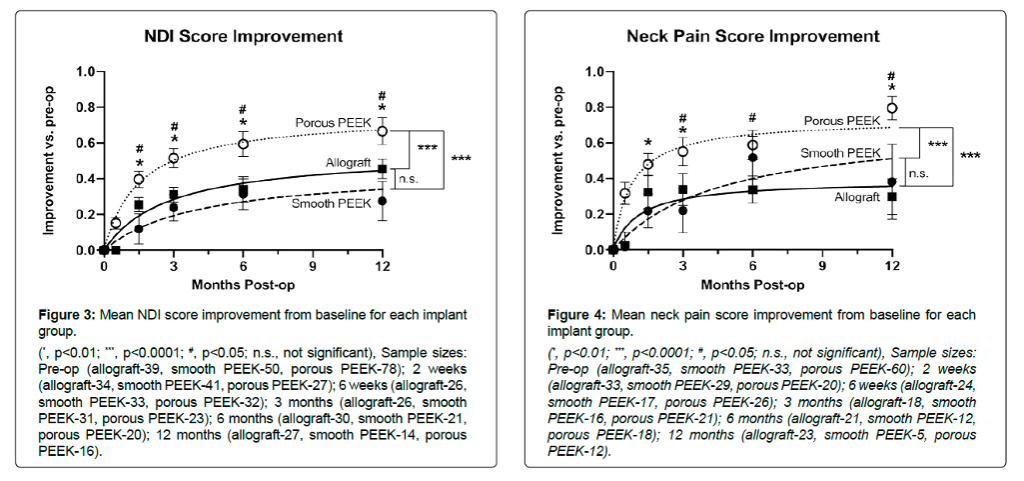
Significance:
In this comparative clinical study, porous PEEK implants demonstrated greater improvements in early clinical outcomes compared to smooth PEEK and structural allograft.
“These data are the first evidence that interbody devices designed to enhance bone in-growth into a porous architecture incrementally and significantly improve clinical outcomes compared to traditional implants”[1], stated Drs. Hill and Strenge.
To see a full copy of the publication, click here. To learn more about Porous PEEK technology, click here.
For complete important safety information, refer to the Cohere IFU found at nuvasive.com/eifu.
References:
1. Hill CP, Strenge KB (2019) Early Clinical Outcomes Comparing Porous PEEK, Smooth PEEK, and Structural Allograft Interbody Devices for Anterior Cervical Discectomy and Fusion. J Spine Neurosurg 8:1.